How does LIMS help laboratories to fulfill ISO 17025:2005?
Guest posting - published with the permission of Siri H. Segalstad
WHAT IS ISO 17025:2005? - Part 1 of 3
ISO 17025 is the laboratory accreditation standard. That is used by testing laboratories (chemistry, mechanical, electrical, and all other testing), and it is also used by calibration laboratories. The company that calibrates and maintains your instruments in the lab, should be accredited to this standard. The standard is from 2005 and is therefore now relatively old. The main difference between the probably better-known ISO 9001 and 17025, is that there are requirements about competence in what the lab does in 17025, while both 9001:2015 and 17025:2006 have requirements for personnel competence.
ISO 17025 has a large number of requirements for keeping records of everything that happens in the lab; everything, that is, that has to do with the technical quality of whatever the lab is doing. There are also requirements in ISO 17025 regarding the management responsibilities, the quality management system, the organization and other non-technical issues. Most of these items cannot be helped by LIMS.
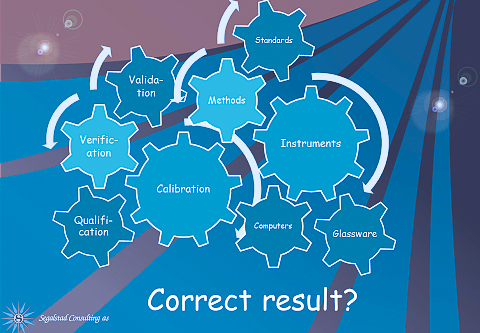
LIMS is the ultimate tool for the laboratory. All the technical requirements in ISO 17025 for lab work can be implemented in LIMS. If so, all information is there at the fingertips of the users when they need it, and not in various logbooks located somewhere in the lab. Here are a few examples, but then list is not complete:
Personnel shall have records of their qualifications in CV, Training records, plans for further qualification training, and job descriptions. They need to be certified for the work they are doing. That is about the same as in ISO 9001.
How LIMS can help: While we normally don’t keep these records inside LIMS, we can let LIMS give access to e.g. analytical methods or instrument that the person has been trained in using. The person without that access cannot use these methods or instruments.
Instruments need to be maintained, calibrated, and shall not be used outside their calibration/maintenance dates.
How LIMS can help: The information on the instrument is implemented as static data in LIMS, which means that this is not normally changed. That is e.g. the name and type of the instrument, calibration and maintenance schedule, connection to LIMS, etc. When we maintain or calibrate the instrument, the dates and other required information are entered. When we then use the instrument, we know if it has been calibrated/maintained or not, and we enter the results from this particular sample. There is no need to keep a paper instrument logbook, and no need to check if the instrument was inside or outside of its calibration period or calibration results, as everything is in LIMS. LIMS can be set up either to accept the use of the instrument outside the rules, and then flag the fact that it is outside, or so that LIMS can refuse the use of the instrument when it is not calibrated/maintained. With instruments connected directly to LIMS, the risk of typos when manually transferring the data from e.g. the lab notebook to LIMS, is eliminated.
Standards, reagents, and other “helpers” in the work also needs to be kept track of. Chemicals are typically made and have an expiration date. There is a need to know the identification of the original, how it has been diluted, etc.
How LIMS can help: We can enter the original with identification, make LIMS calculate the concentration based on the input weight from the balance and the volume for dilution. If we then let LIMS print out a label with bar codes, all we need to do when using the reagent is to scan the barcode. LIMS will then use the information for that reagent for further calculations. The use of the chemical is also connected to the sample where it was used.
Stay tuned for part two, which will be published soon.
Writer

Siri H. Segalstad, Segalstad Consulting AS