How does LIMS help laboratories to fulfill ISO 17025:2005 - PART 3
Guest posting - published with the permission of Siri H. Segalstad.
How does LIMS help laboratories to fulfill ISO 17025:2005? - Part 3 of 3
IMPLEMENTATION
The first one is to implement everything in LIMS. A lot of labs will wait a while with e.g. connecting the instruments to LIMS. That means that a lot of features are not in place, including automatic transfer of data from the instrument to LIMS (and also transfer from LIMS to the instrument to tell the instrument what to do). The risk of typos when we manually type in the values, has been calculated to 100 times larger than when it is an automatic transfer from an instrument or an automatic calculation. The manual entry also means that we will have to do a manual check, which will give a lot more work than when everything has been implemented in LIMS. The LIMS can be implemented so that a lot of things can be done automatically. For example: If all results are within whatever specifications or limits we have decided, there is no need to check anything manually. However, if there are some problems with anything (calibration, expiration, out of limits, etc.), LIMS will flag this so it is easy to see what we need to check it a bit closer before the results are sent to the customer.
VALIDATION
The second one is to validate LIMS. There are two things we need to validate: That the LIMS functions work correctly (that is the performance testing of LIMS). This needs to be revalidated when the system is upgraded from one version to the other.
We also need to validate that all the information we have entered as static data, is correct. Examples: We know that LIMS does correct calculations, but have we entered the correct calculations for this analytical method? The LIMS validation is basically done once and then it is OK until we upgrade LIMS and we need to check it again.
The analytical methods, sample types and information, calculations (inside an analytical method and between methods if needed), need to be validated before the new static data is used. Likewise with reports: LIMS uses a report tool, but we decide which information shall be in the report. The reports will also have to be validated so we know that the correct data is reported, that all references have been entered, all values have been flagged as inside or outside of some specification, and that the report is sent to the right recipient.
There will be no need to manually check that reports are correct, as validation has proven that they do report the correct information. So what about the signature at the bottom of the Certificate of Analysis? That does not have to be handwritten. Personally I do not like to see a scanned handwritten signature, regardless of how this has been made. It looks too fake to me, kind of like the “personal” letters we receive to buy something. I would much rather see the report with a printed name of the authorizer, including the information that this has been authorized electronically. But this is my opinion.
CONCLUSION
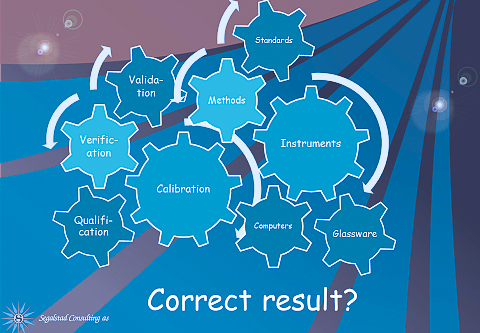
What has an impact on the corrrect result? Calibration, qualification, computers, glassware (for chemistry), instruments, standards, analytical methods, and a lot of other factors. Every single one of these can be implemented in LIMS. If e.g., glassware is identified, there is no reason why we can not enter the verification results of each glassware into LIMS. If we additionally label them with a barcode, scanning them before use will give yet another layer of quality to our results.
ISO 17025:2005 requires the lab to keep and maintain a lot of information around the technical data that it reports. When LIMS has been properly implemented and validated, LIMS will fulfill all these technical requirements. When all the implementation and validation have been done, the ISO 17025-accredited lab can use LIMS and know that LIMS delivers the right results all the time.
REFERENCES
Details about IT system validation can be found in the book
«International IT Regulations and Compliance», author Siri H. Segalstad, Wiley 2008, ISBN 978-0-470-75882-3.
ISO 17025:2005 can be purchased from your local standards organization, or from www.iso.org.
The blog post is a summary based on a 5-day class on ISO 17025, created by Siri H. Segalstad.
Writer

Siri H. Segalstad, Segalstad Consulting AS