Ensure Your Data Integrity With SDMS
The handwriting was always on the wall. (Or perhaps more aptly, the data was always on a storage device.)
In our nearly three decades as a LIMS vendor, the volume & complexity of data we help customers manage has grown exponentially.
The ubiquity of data has led to its importance in business decision-making and clinical/healthcare settings. In fact, in most labs today there are few things as important as data and our collective ability to ensure its accuracy and traceability.
Data Integrity Matters.
We get it: data integrity is important to you!
LabVantage’s focus on data integrity is just one of the many reasons why the new Scientific Data Management System (SDMS) coming in LabVantage 8.5 is so important. But before we can understand why, first we need to understand ALCOA+.
Understanding ALCOA+
ALCOA+ is an acronym, representing: ‘attributable, legible, contemporaneous, original, accurate, consistent, available, enduring, and complete.’ (‘Consistent,’ ‘available,’ ‘enduring’ and ‘complete’ were added later, hence the ‘plus’ sign). ALCOA+ is a set of (now 9) key factors involved in determining whether data can be trusted.

Is Your Data Trustworthy?
“Can I trust my data?”
Sounds like a crazy question, right? Of course you can trust your data…can’t you? Actually, it’s not a stupid question. Manual data entry, for example, can introduce errors. Data not collected in a timely manner may be stale and no longer relevant. Data may be partially collected, leading to incomplete or missing datasets. In such cases, no – you cannot trust your data.
All of the challenges – missing data, erroneous data, old data – are related to the ALCOA+ principles…in these particular instances, ‘accurate,’ ‘contemporaneous’ and ‘complete.’
Attributable Data – Where Did it Come From?
Think about the data that flows into your LIMS system – whether from a file, via an automated interface or by human entry. To ensure the integrity of the data, it’s critical to understand where the data comes from, which instruments or processes produced it, and where it was stored.
When collecting data from these different sources, an SDMS serves as a central repository, not just for the data itself but also for the information about the data (META) – which instrument generated the file, where it was stored, the date & time of the event, and much more.
How Does a SDMS Help Ensure Data Integrity & Security?
Unlike paper records that can be lost, compromised (ever spill coffee on your desk?), or degrade over time, digital copies remain indefinitely unchanged. So first things first: if you haven’t begun your lab’s digital transformation yet, give it some very serious consideration!
Where a LIMS is designed to handle sample data, an SDMS is purpose-built to manage instrument data.
An SDMS allows you to: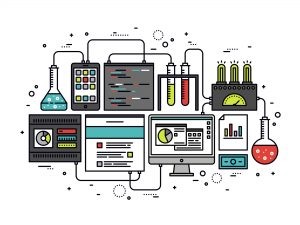
- Send data for SDMS storage, while ensuring its availability to the LIMS, ELN & LES.
- Manage instrument-related data in a repository. Data is immediately collected, tracked, secured, audited on changes, and can be retrieved for further processing.
- Create and maintain instrument drivers in a powerful and industry-standard UI.
When data is collected into the LabVantage SDMS, the collection is nearly immediate. The SDMS can also be adjusted to collect the data faster or slower, based on the urgency in your lab to get the data in question under control. All collected files are digitally copied into the SDMS (constituting what the FDA calls a “true copy”), which ensures the authenticity of the original.
The LabVantage platform uses internal APIs to execute data entry, which avoids relying on passing data through files or other manual mechanisms – any of which could introduce errors. Once a data source driver is created and validated, you can be absolutely certain that the entry of the measured values are accurate and complete.
‘Junk Data In’ Doesn’t Have to Mean ‘Junk Data Out.’
Sometimes the data introduced into the system isn’t formatted correctly. It may contain indecipherable values, given the context. LabVantage’s SDMS immediately sends an alert of the problem so that it can be addressed before data is entered into the system incorrectly.
Putting the Plus in ALCOA+
Two of the later additions to the ALOCA standard – ‘available’ and ‘enduring’ – can be a key concern. There can be numerous stakeholders with an interest in lasting availability of the collected data.
Once captured in LabVantage’s SDMS, data is immediately packaged, encrypted, and placed into the SDMS storage system of your choice – for example, within the LIMS database, or even on an application or file server that is unavailable to your users (or specific groups of users).
 Not only are captured files encrypted to prevent tampering, but digital packages of files are obfuscated. Its encrypted hash is checked every time it is accessed, to ensure digital ‘true copies’ have not been tampered with and are guaranteed to be complete.
Not only are captured files encrypted to prevent tampering, but digital packages of files are obfuscated. Its encrypted hash is checked every time it is accessed, to ensure digital ‘true copies’ have not been tampered with and are guaranteed to be complete.
Data remains in the LabVantage SDMS for as long as you desire. It ensures that you will always be able to trust your data when it comes to future audits, analytics, or when performing other data-related tasks.
Data Integrity: A Purpose-Built SDMS
When it comes to data integrity and security, leveraging the capabilities of an SDMS offers many advantages:
- Immediately capture externally generated data, whether from complex instruments, real-time data sources or incoming emails and other file captures (e.g., local drives, file shares).
- Ensure security and control against data loss or modification.
- Use powerful graphical parsers or Talend-based drivers to import lab data.
- Deploy remote file collectors to reduce data bottlenecks.
- Utilize robust storage options, including the LIMS file system and database.
- Quickly link back to files, including delimited, Word, Excel, text, PDF, and more.
LabVantage’s SDMS integrates seamlessly with instruments and data sources, capturing and securing even the most complex data.
Learn more about LabVantage’s fully-integrated laboratory software suite with LIMS, ELN, LES and SDMS capabilities