Can a Pharma LIMS Really be Pre-Validated?
We often field questions about the LabVantage Pharma Accelerator, which is described as a ‘pre-validated’ platform. 
But what does ‘pre-validation’ actually mean?
Quality and computer system validation professionals understand that formal validation can only take place on the production system, or a system that is identically configured, using the target lab’s intended use, actual product definitions, instruments and workflows.
In short, it must either be the real thing or a reasonably identical duplicate.
Basic common sense suggests that these conditions simply aren’t available when LabVantage validates its pharma product – and yes, this is in fact the case.
LabVantage performs testing on a representative system that replicates many of the most common characteristics of pharma labs, using ad hoc selected product definitions. Having worked with pharma labs for decades, we have a deep knowledge of both workflows and regulatory compliance in the space. Creating a representative system to test our accelerator isn’t a marketing gimmick; bio/pharma is deep-rooted in the LabVantage ethos.
But that’s not the same thing as validating the software in your lab using the precise products, devices and methods you deal with every day.
So what do we mean by ‘pre-validated?’ Isn’t it impossible to buy lab software that is already validated?
The short answer is yes — you can’t purchase LIMS software that is already validated, and you must validate all lab software in-house with your own systems and tools.
But we can help you streamline the process. The key to understanding our use of the word “pre-validated” is to get to the bottom of how the prefix “pre-” is used, and its specific meaning in this context.
 A Short ‘Pre-amble’
A Short ‘Pre-amble’
The prefix “pre-” can mean several things, but in relation to the LabVantage Pharma Accelerator, it means “beforehand”, which implies both “previously” and “already.”
In terms of validation, these are two very different things:
- On the one hand, it is very helpful to review evidence of previous
- On the other, it is impossible to purchase software that is already 100% validated.
Therefore, LabVantage Pharma is previously validated, but not already validated for your lab.
When validating software (or hardware, or other instrumentation and processes), there are three types of validation and qualification tasks required. These are:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
IQ and OQ are evaluated to determine that the system meets the installation and operating parameters established by the manufacturer (in this case, LabVantage).
PQ must be performed in the company’s actual operating environment to ensure the viability of the system under real-world conditions.
Hamming It Up: A Pre-Validation Analogy
Let’s consider an example from the real world: a pre-cooked ham.

We all know ham can’t be eaten raw so we typically buy it pre-cooked. And…once you get your ham home, you can slice off a piece of your pre-cooked ham and eat a ham sandwich. It’s a quick, easy and safe meal because the ham was previously baked.
But that doesn’t mean your hypothetical ham is already heated. You can toss it in cold between two slices of bread and add whatever condiments you like, but it’s not hot right now.
Now, if you came to my house for a holiday dinner and I served you cold ham, it would be…underwhelming, to say the least. That’s because in a holiday setting, you expect your ham to be served hot with a little extra effort. Under those circumstances, I would put some pineapple on it, add some glaze, bake it in the oven and serve it heated.
As odd as it sounds, LabVantage Pharma is similar to a pre-cooked ham: “previously” validated (heated) but not “already” validated (hot). But what does this mean for you?
Why Pre-Validation Matters
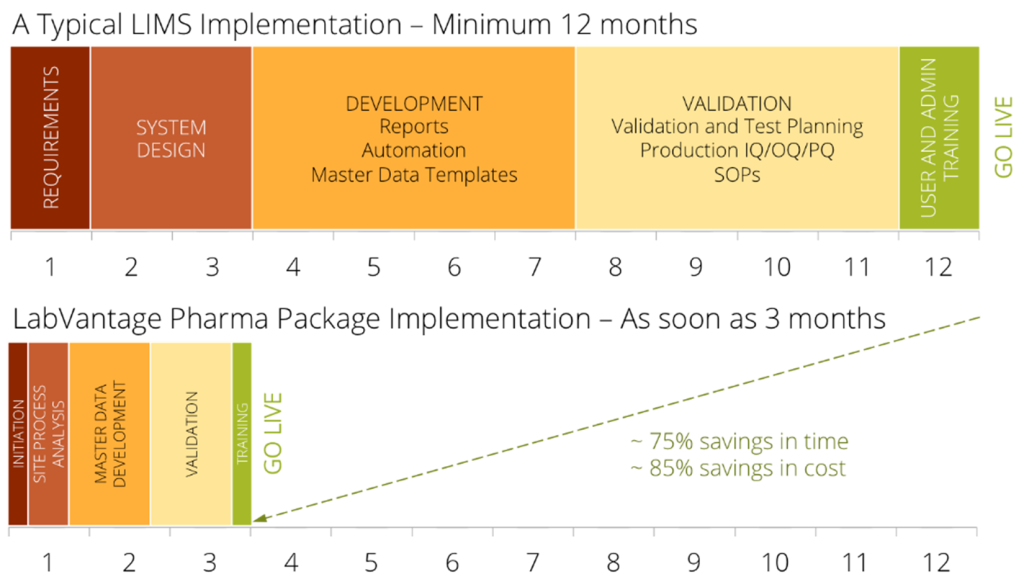
Half-serious musings about mouthwatering pork products aside, pre-validation offers your pharma lab significant advantages. First, it dramatically reduces your deployment time because we’ve done much of the up-front work for you. You’ll still have to do the validation process (PQ) for your own lab, but it will typically take you about a quarter of the time compared to a traditional LIMS implementation.
 On average, you’ll also save around 85% on deployment costs. (Yes, that’s a lot of ham!)
On average, you’ll also save around 85% on deployment costs. (Yes, that’s a lot of ham!)
One huge benefit of a pre-validated platform is that users can take advantage of LabVantage’s decades of experience implementing LIMS in the pharma industry. This includes out-of-the-box workflows and the functionalities required by pharma and biotech labs – from batch management and stability testing to consumables management, environmental monitoring, barcode label printing, and more.
No other vendor has comparable expertise, which is why LabVantage Pharma is the world’s only pre-validated and pre-configured pharmaceutical LIMS.
Have questions about LabVantage Pharma or evidence of previous validation? Reach out to us. We’ll be happy to walk you through the process and explain how this package can expedite your implementation and validation activities.